Have you ever wondered about some of the less obvious things in your everyday world, perhaps something that shows up in your food, your body, or even some common products? It's a pretty interesting thought, you know. Today, we're going to take a closer look at something called glycerol, a simple compound that, as a matter of fact, plays a surprisingly big part in lots of different areas. It’s a substance that’s been getting a bit more attention lately, especially when we talk about what goes into some of our favorite treats, so it's worth getting to know a little more about it.
This sweet-tasting, clear liquid might seem, you know, pretty unassuming at first glance. Yet, it's really a key player in how our bodies work, how certain products are made, and even, apparently, in some discussions about public well-being. From its fundamental chemical makeup to its roles in biological processes and various industrial applications, glycerol, or what some might call trihydroxypropane, has quite a story to tell. We'll explore what it is, where it comes from, and why it matters, so stick around.
We'll cover its definition, how it helps our bodies, and some of the ways it's used out in the world, like in things you might eat or even in medicines. You'll also learn about some important considerations, especially when it comes to children and certain types of drinks. It's a really versatile compound, and understanding it can give you a better picture of how many things around us function, or so it seems.
Table of Contents
- What is Glycerol?
- Glycerol in Our Bodies: A Key Energy Player
- More Than Just Energy: Other Roles for Glycerol
- Glycerol in Everyday Products
- Glycerin vs. Glycerol: Is There a Difference?
- When Glycerol and Other Substances Mix
- Important Considerations and Warnings
- Frequently Asked Questions About Glycerol
What is Glycerol?
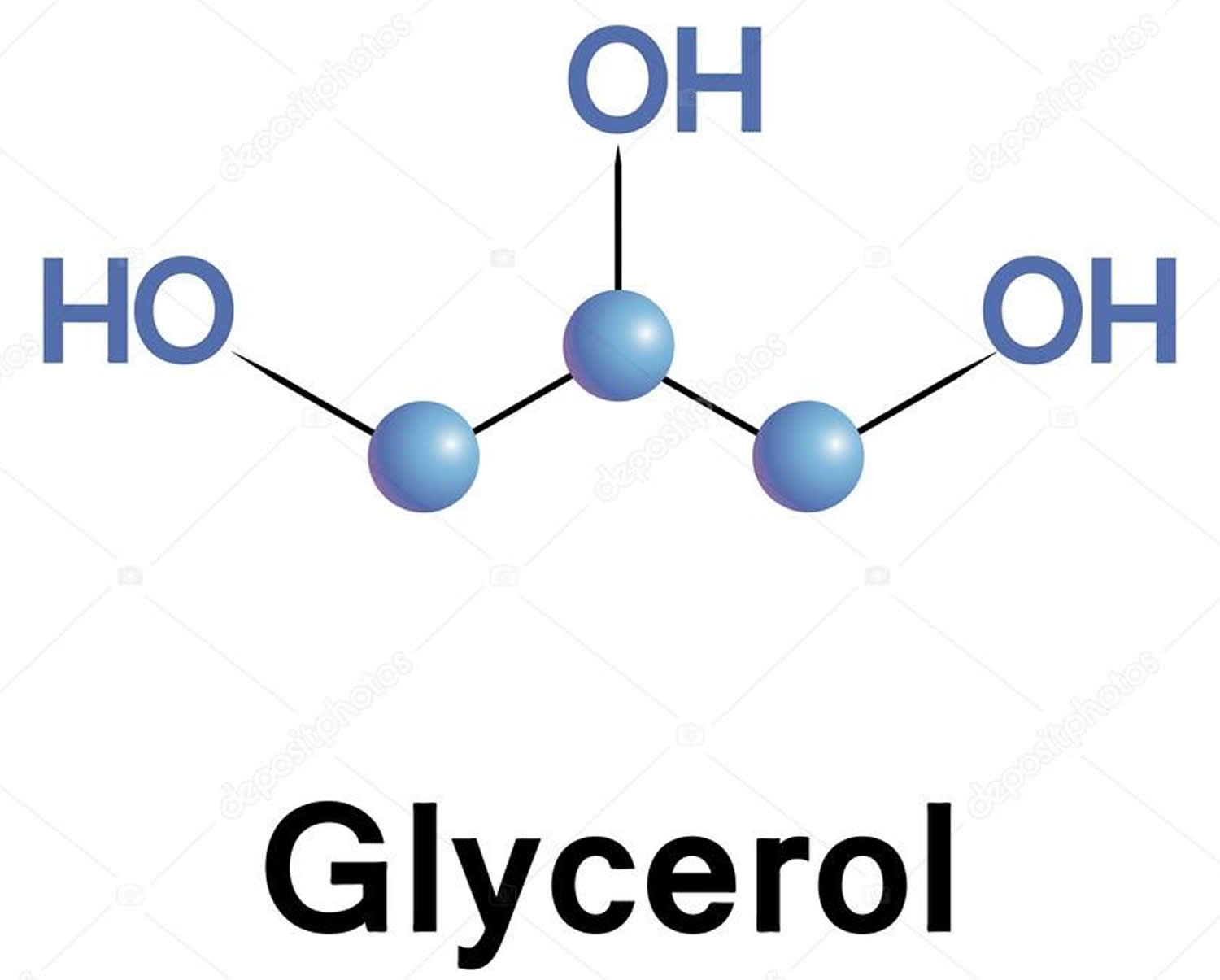
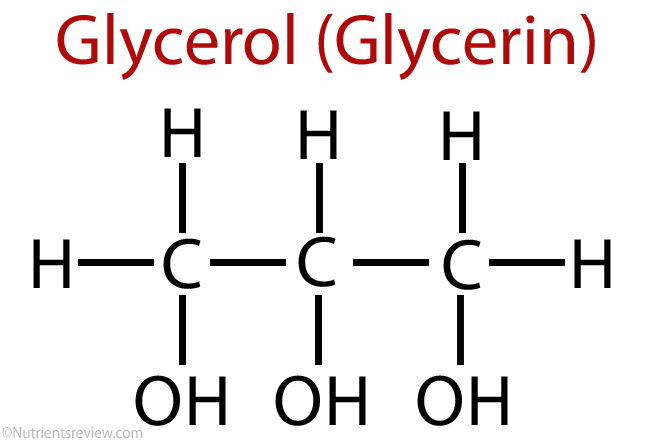
So, what exactly is this stuff we call glycerol? Well, to put it simply, it's a colorless liquid that doesn't really have a smell, and it tastes a bit sweet. It's classified as a simple polyol compound, which basically means it's an organic molecule with several hydroxyl groups attached. Think of it as a propane structure, but with a special kind of alcohol group on each of its three carbon atoms, you know, at positions 1, 2, and 3. This unique structure is what gives it many of its interesting properties, and it's pretty much why it can do so many different things.
Its chemical name, trihydroxypropane, really spells out what it is: three hydroxyl groups on a propane backbone. This structure, you know, makes it quite water-friendly. Water, as a matter of fact, mixes completely with glycerol. This ability to mix with water is a pretty big deal for many of its uses, whether it's in your body or in something you buy at the store. It's a very common molecule in nature, found in all living things, which is kind of cool when you think about it.
Glycerol in Our Bodies: A Key Energy Player
When we talk about how our bodies work, glycerol has some really important jobs, particularly when it comes to fats and energy. You see, glycerol is a starting material, a "precursor" as some might say, for making triacylglycerols and phospholipids. These are both kinds of fats, or lipids, that our bodies need for all sorts of things. Triacylglycerols are basically how our bodies store energy, like a personal fuel tank, and phospholipids are, you know, the main building blocks for the walls of our cells. Our liver and adipose tissue, which is where we keep our stored fat, are the main places where these processes happen.
It's pretty fascinating how our bodies handle energy, isn't it? When we need a burst of power, or just to keep things running, our bodies often turn to stored fat. When this happens, the stored fat gets broken down, and guess what? Glycerol is one of the things that comes out of that process, along with fatty acids. So, in a way, glycerol is a piece of the energy puzzle, a part of how we get fuel from what we've saved up. This makes it, you know, quite important in various metabolic pathways, especially those that have to do with lipids, or fats, in our system.
This compound also acts as an osmolyte, which is a fancy way of saying it helps cells manage their water balance. Imagine a cell trying to keep the right amount of water inside, not too much and not too little. Glycerol helps with that, sort of like a tiny water manager. It’s pretty crucial for keeping our cells working as they should, you know, making sure they don't dry up or swell too much, especially when there's a lot of salt around. If there's too much salt, water tends to flow out of the cell, and glycerol can help mitigate that, basically.
More Than Just Energy: Other Roles for Glycerol
Beyond its significant roles in our own bodies, glycerol is, you know, quite a versatile compound with a lot of different uses in the wider world. It's a common ingredient in many products you might use every day. For instance, it can act as a solvent, meaning it can dissolve other substances, which is pretty handy in making various solutions. It's also used as a detergent, helping things get clean, which is a very practical application, obviously.
Think about some of the things that need to stay soft and flexible, like certain plastics or even some food items. Glycerol can act as a plasticizer. What's a plasticizer, you ask? Well, it's an additive that, as its name sort of hints at, helps to make a plastic more bendy and less brittle. It works by, you know, getting in between the molecules of the material and helping them slide past each other more easily. In food, it helps keep things like starch stretched out and stable, often by forming hydrogen bonds, which is a neat trick.
It also shows up as a human metabolite, meaning it's a substance that's produced or used when our bodies process things. And, surprisingly, it's even an algal metabolite, so tiny water plants make it too! This just goes to show how fundamental and widespread this simple molecule really is in the natural world, pretty much everywhere you look, in a way.
Glycerol in Everyday Products
You might be surprised at just how often you encounter glycerol in products you use or consume. Because of its sweet taste and its ability to attract and hold moisture, it's a common additive in many food and drink items. It helps keep things moist, prevents them from drying out too quickly, and can add a pleasant sweetness without being sugar, which is, you know, a pretty good benefit for some products. It's also found in many personal care items, like soaps, lotions, and even toothpaste, where it helps with texture and moisture retention.
In the pharmaceutical world, glycerol is used in a variety of medicines. It can be a solvent for drug formulations, helping to dissolve active ingredients so they can be delivered effectively. It's also used in some cough syrups and laxatives, where its properties help achieve the desired effect. So, you know, it's not just a food additive; it's a pretty important component in health products too. Its versatility really makes it a go-to ingredient for manufacturers, as a matter of fact.
Glycerin vs. Glycerol: Is There a Difference?
This is a question that comes up quite a bit, and it can be a little confusing for people, you know, because there seems to be some conflicting information out there. Is there a difference between glycerin and glycerol, especially when you're in a laboratory setting or just talking about them generally? The short answer is, not really in a chemical sense. Glycerol is the pure chemical compound, the specific molecule we've been talking about with its precise structure. Glycerin, on the other hand, is the more common name used for the commercial product, which is typically a highly purified form of glycerol, usually at least 95% pure.
So, when you see "glycerin" listed as an ingredient in your hand cream or a food item, it's basically glycerol. The terms are often used interchangeably, but "glycerol" is the chemical term, and "glycerin" is the common name for the purified substance used in products. It's a bit like how "sodium chloride" is the chemical name for "table salt." They refer to the same thing, just in different contexts, pretty much. So, you know, if you hear one or the other, they're likely talking about the same useful compound.
When Glycerol and Other Substances Mix
Glycerol has some interesting interactions with other chemicals, which is, you know, part of what makes it so useful. We already mentioned that water mixes completely with glycerol. This is because both water and glycerol can form hydrogen bonds, which helps them blend together really well. However, not everything mixes with it. For example, according to the CRC Handbook, glycerol and diethyl ether are immiscible, meaning they don't mix, similar to how water and ether don't mix. This property of mixing with some things but not others is pretty important for how it's used in different solutions and products.
People sometimes wonder about its reactions with acids. For instance, what happens if glycerol and citric acid are combined, especially if they're heated to a high temperature? Well, glycerol can react with acids, forming compounds called esters. The specific outcome depends on the conditions, like the temperature and the presence of catalysts. It's a pretty common reaction in organic chemistry, and it's how some fats are formed naturally, too. You know, these kinds of reactions are fundamental to many industrial processes.
There's also an interesting point about its acidity. If the pKa of glycerol is 14.15, how do you calculate the pH for it? This is a question for chemistry buffs, but basically, its high pKa value means it's a very weak acid, so it doesn't readily give up its protons in water. However, its properties can change when it interacts with other compounds. For example, boric acid, which is a weak acid on its own, becomes much stronger when certain organic polyhydroxy compounds, like mannitol, dextrose, or indeed glycerol, are added to it. This interaction, you know, is pretty neat and has practical uses in analytical chemistry, as a matter of fact.
Someone once boiled a solution of glycerol and water at about 160 degrees Celsius and ended up with a brown liquid that was much less in volume. This probably meant that most, if not all, of the water boiled off, leaving behind a more concentrated, possibly slightly caramelized glycerol. Glycerol has a much higher boiling point than water, so it would remain behind. This kind of observation, you know, really shows how different substances behave when heated, and it's pretty much a common experience in the kitchen or laboratory.
Important Considerations and Warnings
While glycerol is widely used and generally considered safe in many applications, there are some important things to be aware of. Recently, experts have raised concerns about certain products containing glycerol, particularly for young children. For instance, there have been warnings that slushies, those flavored drinks made of syrup and crushed ice, that contain the additive glycerol can make children below the age of eight very ill. This is, you know, a pretty serious warning, and it highlights the need to be mindful of what our little ones are consuming.
It's always a good idea to, you know, be aware of the ingredients in products, especially those marketed to children. While glycerol itself has many beneficial uses and is a natural part of our bodies, its concentration and how it's consumed can sometimes lead to problems for sensitive systems. So, learning more about glycerol uses, its effectiveness, possible side effects, and recommended dosages is always a smart move. You can find more information about its uses and safety by checking out reliable sources, like the kind of detailed information you might find on a reputable health website or, you know, a government health agency site, for example, WebMD's page on glycerol.
Understanding the properties of substances like glycerol helps us make more informed choices, especially regarding what we put into our bodies or give to our families. It's a really interesting compound, and its roles are, you know, pretty diverse, from being a human metabolite to a plasticizer. So, next time you see it listed as an ingredient, you'll have a much better idea of what it is and what it does. You can learn more about glycerol on our site, and also find more information on this page here.
Frequently Asked Questions About Glycerol
What is the main role of glycerol in the body?
Basically, glycerol is a key building block for making fats, like triacylglycerols and phospholipids, which are important for storing energy and building cell walls. It's also, you know, a part of how our bodies get energy when they break down stored fat, so it's pretty central to how we use fuel.
Is glycerol safe to consume, especially for children?
While glycerol is generally used in many products, there have been warnings about its use in high amounts, especially in drinks like slushies for young children under eight years old. Experts have, you know, suggested it can make them quite ill, so it's always good to be cautious and check ingredients.
Can glycerol be used in industrial applications?
Absolutely! Glycerol is, you know, quite versatile. It's used as a solvent, a detergent, and even as a plasticizer to make materials more flexible. It has a lot of different uses in manufacturing and other industries, as a matter of fact.

Detail Author:
- Name : Mr. Keith Ledner
- Username : kulas.melody
- Email : sauer.fred@yahoo.com
- Birthdate : 1979-12-31
- Address : 9314 Lavon Parks East Beaulahton, NE 46270-5940
- Phone : 863.629.8929
- Company : Bauch-Ziemann
- Job : Graphic Designer
- Bio : Nisi ut voluptas consequatur cumque beatae voluptate. Ipsum voluptas voluptas et beatae qui commodi est. Quo nemo commodi optio cumque. Hic iusto sed at.
Socials
facebook:
- url : https://facebook.com/abel_dev
- username : abel_dev
- bio : Sed eaque in libero consequatur blanditiis saepe.
- followers : 4880
- following : 219
instagram:
- url : https://instagram.com/abel_lindgren
- username : abel_lindgren
- bio : Dolores porro vel soluta nesciunt officia. Nam et vero consequatur ea similique quaerat et.
- followers : 1354
- following : 2237
linkedin:
- url : https://linkedin.com/in/abellindgren
- username : abellindgren
- bio : Dolorem inventore totam est temporibus.
- followers : 1027
- following : 40

